Colloidal systems are extremely important in the life of any person. This is due not only to the fact that almost all biological fluids in a living organism form colloids. But many natural phenomena (fog, smog), soil, minerals, food, medicines are also colloidal systems.

The unit of such formations, reflecting their composition and specific properties, is considered to be a macromolecule, or micelle. The structure of the latter depends on a number of factors, but it is always a multilayer particle. Modern molecular kinetic theory considers colloidal solutions as a special case of true solutions, with larger particles of the dissolved substance.
Methods for obtaining colloidal solutions
The structure of the micelle formed when a colloidal system arises partly depends on the mechanism of this process. Methods for producing colloids are divided into two fundamentally different groups.
Dispersion methods involve grinding fairly large particles. Depending on the mechanism of this process, the following methods are distinguished.
- Grinding Can be done dry or wet. In the first case, the solid is first crushed and then the liquid is added. In the second case, the substance is mixed with a liquid, and only after that it is turned into a homogeneous mixture. Grinding is carried out in special mills.
- Swelling. Grinding is achieved due to the fact that solvent particles penetrate into the dispersed phase, which is accompanied by the moving apart of its particles up to separation.
- Ultrasonic dispersion. The material to be crushed is placed in a liquid and treated with ultrasound.
- Dispersion by electric current. Demanded for the production of metal sols. It is carried out by placing electrodes made of dispersible metal in a liquid and then applying high voltage to them. The result is a voltaic arc in which the metal is atomized and then condensed into a solution.
These methods are suitable for the preparation of both lyophilic and lyophobic colloidal particles. The structure of the micelle occurs simultaneously with the destruction of the original structure of the solid.

Condensation methods
The second group of methods, based on the enlargement of particles, is called condensation. This process may be based on physical or chemical phenomena. Methods of physical condensation include the following.
- Replacing the solvent. It comes down to transferring a substance from one solvent, in which it dissolves very well, to another, in which the solubility is much lower. As a result, small particles will combine into larger aggregates and a colloidal solution will appear.
- Condensation from vapors. An example is fog, particles of which can settle on cold surfaces and gradually become larger.
Methods of chemical condensation include some chemical reactions accompanied by precipitation of a complex structure:
Conditions for chemical condensation
The structure of micelles formed during these chemical reactions depends on the excess or deficiency of the substances involved in them. Also, for the appearance of colloidal solutions, a number of conditions must be met to prevent the precipitation of a sparingly soluble compound:
- the content of substances in the mixed solutions should be low;
- the speed of their mixing should be low;
- one of the solutions must be taken in excess.

Micelle structure
The main part of the micelle is the core. It is formed by a large number of atoms, ions and molecules of an insoluble compound. Usually the core is characterized by a crystalline structure. The surface of the core has a reserve of free energy, which allows it to selectively adsorb ions from the environment. This process obeys Peskov’s rule, which states: on the surface of a solid, those ions that are capable of completing its crystal lattice are predominantly adsorbed. This is possible if these ions are related or similar in nature and shape (size).
During adsorption, a layer of positively or negatively charged ions, called potential-determining ones, is formed on the micelle core. Due to electrostatic forces, the resulting charged aggregate attracts counterions (ions with opposite charges) from the solution. Thus, the colloidal particle has a multilayer structure. The micelle acquires a dielectric layer built from two types of oppositely charged ions.
Hydrosol BaSO4
As an example, it is convenient to consider the structure of a barium sulfate micelle in a colloidal solution prepared in an excess of barium chloride. This process corresponds to the reaction equation:
Barium sulfate, slightly soluble in water, forms a microcrystalline aggregate built from the m number of BaSO molecules4. The surface of this aggregate adsorbs the nth number of Ba 2+ ions. There are 2(n - x) Cl - ions associated with the layer of potential-determining ions. And the rest of the counterions (2x) are located in the diffuse layer. That is, the granule of this micelle will be positively charged.
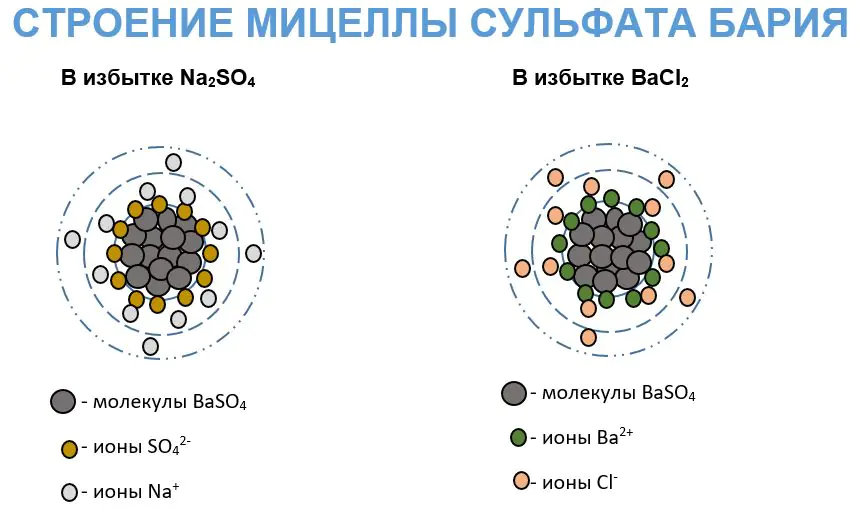
If sodium sulfate is taken in excess, then the potential-determining ions will be SO ions4 2-, and counterions – Na +. In this case, the charge of the granule will be negative.
This example clearly demonstrates that the sign of the charge of a micelle granule directly depends on the conditions of its preparation.
Recording a micelle
The previous example showed that the chemical structure of micelles and the formula that reflects it are determined by the substance that is taken in excess. Let's consider ways of writing the names of individual parts of a colloidal particle using the example of copper sulfide hydrosol. To prepare it, sodium sulfide solution is slowly poured into an excess amount of copper chloride solution:

Structure of a CuS micelle obtained in an excess of CuCl2, is written as follows:
Structural parts of a colloidal particle
The formula of a sparingly soluble compound, which is the basis of the entire particle, is written in square brackets. It is usually called an aggregate. Usually the number of molecules that make up an aggregate is written with the Latin letter m.
Potential-determining ions are contained in excess quantities in the solution. They are located on the surface of the unit, and in the formula they are written immediately behind square brackets. The number of these ions is denoted by the symbol n. The name of these ions indicates that their charge determines the charge of the micelle granule.
The granule is formed by the core and part of the counterions located in the adsorption layer. The magnitude of the granule charge is equal to the sum of the charges of potential-determining and adsorbed counterions: +(2n – x). The remaining part of the counterions is in the diffuse layer and compensates for the charge of the granule.
If Na was taken in excess2S, then for the resulting colloidal micelle the structure diagram would have the form:
Surfactant micelles
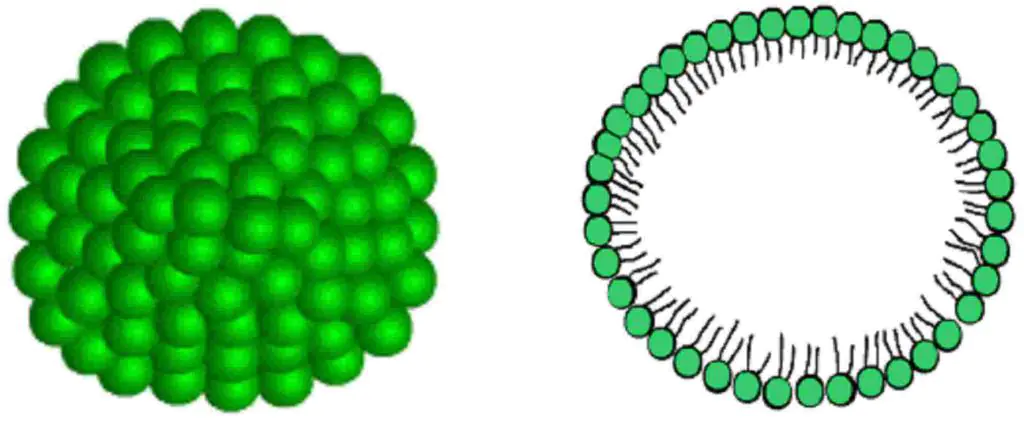
If the concentration of surfactants (surfactants) in water is too high, aggregates of their molecules (or ions) may begin to form. These enlarged particles have the shape of a sphere and are called Hartley-Rehbinder micelles. It is worth noting that not all surfactants have this ability, but only those with an optimal ratio of hydrophobic and hydrophilic parts. This ratio is called hydrophilic-lipophilic balance. The ability of their polar groups to protect the hydrocarbon core from water also plays a significant role.
Aggregates of surfactant molecules are formed according to certain laws:
- unlike low-molecular substances, the aggregates of which can include a different number of molecules m, the existence of surfactant micelles is possible with a strictly defined number of molecules;
- if for inorganic substances the start of micelle formation is determined by the solubility limit, then for organic surfactants it is determined by the achievement of critical concentrations of micelle formation;
- First, the number of micelles in the solution increases, and then their sizes increase.
Effect of concentration on micelle shape
The structure of surfactant micelles is influenced by their concentration in solution. When certain values are reached, colloidal particles begin to interact with each other. This causes their shape to change as follows:
- the sphere turns into an ellipsoid and then into a cylinder;
- a high concentration of cylinders leads to the formation of a hexagonal phase;
- in some cases, a lamellar phase and a solid crystal (soap particles) appear.
Types of micelles
Based on the characteristics of the organization of the internal structure, three types of colloidal systems are distinguished: suspensions, micellar colloids, and molecular colloids.
Suspensoids can be irreversible colloids, as well as lyophobic colloids. This structure is characteristic of solutions of metals, as well as their compounds (various oxides and salts). The structure of the dispersed phase formed by suspensions does not differ from the structure of the compact substance. It has a molecular or ionic crystal lattice. The difference from suspensions is their higher dispersion. Irreversibility is manifested in the ability of their solutions after evaporation to form a dry precipitate, which cannot be converted into a sol by simple dissolution. They are called lyophobic because of the weak interaction between the dispersed phase and the dispersion medium.
Micellar colloids are solutions whose colloidal particles arise from the adhesion of diphilic molecules containing polar groups of atoms and non-polar radicals. An example is soaps and surfactants. The molecules in such micelles are held together by dispersion forces. The shape of these colloids can be not only spherical, but also lamellar.
Molecular colloids are quite stable without stabilizers. Their structural units are individual macromolecules. The shape of a colloid particle can vary depending on the properties of the molecule and intramolecular interactions. So a linear molecule can form a rod or a coil.
What is micellar water? It is essentially a colorless makeup remover. Micellar solution was previously used to care for the skin of babies and treat eczema. The composition was then further developed by cosmetics companies, creating a modern product designed to cleanse the skin.
Micelles - what are they?

So, let’s answer in detail the question, what is micellar water? It got its name from micelles - particles that are formed from surfactants (in other words, surfactants) when their concentration in water reaches a certain level.
Micellar products include foam for washing, various cleansers, etc. When micelles get into water, the lipophilic components are attracted to each other, staying away from the water. Micelles attract both fat particles and cosmetic residues, enveloping them.
Surfactants serve as a kind of bridges from water to fats. From their molecules, components are formed in the form of spherical crystals. They have lipophilic (fat) tips that point towards the center, and hydrophilic ones that tend to reach the water molecule.
To complete the review of the issue, what is micellar water, we note that micelles are characterized by the following features:
- reduce the irritating properties of cleansers. Paradoxically, irritation from sodium lauryl sulfate is greater if it is contained in a low concentration in the cleanser (insufficient to form micelles);
- deactivate some substances. The formation of micelles occurs around molecules, i.e. an obstacle to the toxic effects of ingredients is created. However, the downside is that they can inhibit substances beneficial to the skin, and cosmetic products may not give the predicted result;
- wash away the smallest particles of fat, which is why micellar water is the optimal makeup remover.
Composition of micellar water
Micellar water may contain:
- purified water. Melt and glacial water soothe and nourish the dermis, their effect is very gentle. The thermal liquid contains many minerals, which is why it is more effective than the first two types. Flower water is obtained by processing plants; it tones, cleanses, helps with problem skin, and moisturizes dry dermis. Sea water - an analogue of blood plasma - soothes, has a bactericidal effect, relieves swelling, smoothes out a fine network of wrinkles;
- hydrolates. They are actually herbal tinctures. They have a healing effect and contribute to the healing of the epidermis;
- vegetable oils. They should be selected depending on the properties and type of skin;
- tensins. Plant surfactants. They create an obstacle to the adhesion of fat molecules so that a sediment does not form and the suspension is stable;
- glycerin and panthenol. These 2 components have a healing effect, moisturize the skin, and prevent the feeling of tightness. You should choose a product in which glycerin is of plant origin and not synthetic;
- plant extracts, aloe. For normal and oily skin, dispersed water with rosemary, lavender, calendula, mint, and citrus fruits is suitable. For dry and hypersensitive people, the ideal option would be water with the addition of chamomile, aloe vera, ginseng, jasmine, rose, sage, and thyme. Mature skin will benefit from micellar with extracts of mallow, lotus, cornflower, rose, linden, and cedar.
Types of means
There are 3 types of micellar water depending on its base.
- Based on “green chemistry”. They are made from nonionic surfactants (lauryl glucoside, cocoglucoside, etc.), which highly effectively bind particles of dirt and sweat without damaging the skin. Contains sugar and coconut oil.
- Based on poloxamers. These synthesized substances (poloxamer 184, 188, 407, etc.) are perfectly soluble and interact with fats. This micellar does not need to be washed off.
- Based on polyethylene glycol. PEG is considered a classic emulsifier that interferes with the separation of oil and water. If its concentration does not exceed 20%, it is safe, but it can lead to irritation and dryness of the skin, leading to contact dermatitis.
Indications and contraindications
This article provides a detailed answer to the question of what micellar water is made from. What is it used for? Its scope of application is due to the following advantages.
- It has a beneficial effect on the epidermis, saturating it with useful substances.
- Cleanses pores of impurities and waste products of the sebaceous glands, minimizing the risk of acne.
- Micellar water can help remove makeup and correct it (by moistening a cotton swab in it, you can easily remove excess cosmetics, including waterproof ones).
- Characterized by a high level of hypoallergenicity.
- It is a good moisturizer and prevents skin from drying out.
- Helps relieve irritation and heal microcracks.
The following conditions are considered contraindications for the use of micellar water.
- An allergic reaction to a certain type of plant contained in the solution.
- Oily skin prone to breakouts. Some types of dispersed water contain silicone; it covers the skin with a film, preventing oxygen from reaching it.
- Pregnancy. The product sometimes contains preservatives and fragrances, so it is not recommended for pregnant and lactating women to use this micellar to remove makeup.
- Greater skin sensitivity. If the composition contains artificially synthesized glycerin and bromides, peeling and itching of the skin is possible.
How to choose micellar water
Skin sensitivity to various cosmetics is an individual factor. Therefore, before purchasing a large bottle, it is advisable to purchase a sample. Sometimes a negative reaction to the product may appear after 2-3 weeks of use.
You should consider which brand of products you use when caring for your facial skin daily. Cosmetologists recommend purchasing a dispersion solution of the same series to minimize the risk of conflict between formulas.
Also pay attention to the volume of the bottle. Most high-quality micellar solutions can be stored for no more than six months after opening the bottle. Analyze how much skin care liquid you can use during this period.
Features of using micellar water
To remove makeup, thoroughly moisten a cotton swab or disk with micellar water, and then carefully wipe your face along the massage lines. To remove eyeshadow and mascara from your eyelids, apply a damp cotton pad to them for a few seconds without rubbing the liquid. Change the cotton swab and repeat this several times. When it remains clean, the procedure can be considered complete.
Popular brands
Let's look at several popular premium and budget cosmetics.
Premium class
- L’Oreal Paris “Absolute Tenderness” micellar water has a gentle effect and cleanses the skin even from waterproof cosmetics, is suitable for removing eye makeup, and is not addictive.
- Sebium, Sensibio and Hydrabio H2O from Bioderma are suitable for skin that is prone to irritation, for the treatment of acne and blackheads. These pharmaceutical products are based on fruit extract and micelles.
- Normaderm Micellar Solution from Vichy and Posay Physiological Micellar Solution from La Roche usually do not cause negative reactions, they moisturize and cleanse well, and they contain natural ingredients.
A budget option
- Micellar solution “3 in 1” from the company “Clean Line” is a Russian analogue of a foreign product. It contains extracts of rose petals and chamomile, so it has antibacterial and antioxidant properties.
- BIO AQUA produces several types of budget micellar water with vitamins, plant extracts and mineral supplements.
- Nivea 3-in-1 micellar water is a natural product for normal to combination skin that contains vitamin E to help nourish and moisturize. It contains no silicones, parabens or fragrances, natural composition.
- Skin Naturals from Garnier does not cause allergies, moisturizes the skin well, and absorbs oil like an exfoliant. Suitable for dry, depleted and combination skin.
Useful tips
Micellar water is necessary in the following cases.
- If you don’t have time to remove makeup before going to bed. After purchasing micellar water, this problem can be solved - the procedure for removing makeup from the face will take 10-20 seconds.
- For sensitive skin. Micelles have a mild cleansing effect, which compares favorably with products that contain soap. Micellar wash is suitable for removing makeup around the eyes. 3-in-1 products usually contain panthenol to soothe and heal the skin.
- If you are concerned about the environmental friendliness of cosmetics, you can rest assured: a high-quality solution does not contain silicone, parabens or fragrances.
- If you strive for minimalism when purchasing cosmetics. Micellar water will not replace full-fledged care, but it can cope with cleansing, moisturizing and regenerating the skin.
- If you are looking for a universal skin cleanser, you should consider that cosmetologists assure: micellar is suitable for the face (including eyes and lips), neck and décolleté.
Conclusion
So, we have looked at the question of what micellar water is. Before you make a purchase, study the composition indicated on the label so that you make an informed choice, especially if you suffer from dermatitis.
Happy shopping! Let your well-groomed skin shine with purity, and your face with happiness as often as possible!
We love you so much and appreciate your comments that we are ready to donate 3,000 rubles every month. (by phone or bank card) to the best commentators of any articles on our website (detailed description of the competition)!
- Leave a comment on this or any other article.
- Look for yourself in the list of winners on our website!
Return to the beginning of the article or go to the comment form.
4 comments
A new cosmetic product in the beauty industry – micellar water – is becoming increasingly popular among women all over the world. What kind of product is this, what is its composition and how to use it correctly in daily facial care - these are the key questions that interest women of all ages.
Micellar water (as this type of cleanser is lovingly called) or micellar water is a liquid that has a special composition and is intended for the delicate and at the same time effective removal of cosmetics and impurities from the skin of the face. The product does not contain alcohols, synthetic solvents, petrochemical derivatives and soap agents traditionally used in makeup removers.
Good micellar water contains no parabens, no alkalis, no silicones, no dyes, no fragrances. But cosmetics manufacturers, striving to produce high-quality products that maximally improve the condition of the skin, add extracts of flowers and medicinal plants to the product, which have a beneficial effect on the epidermis.
This versatile product is suitable for all skin types and ages and helps dissolve makeup from all areas, including the delicate eye area, without irritating the tear glands. According to the manufacturers' instructions, the product does not require rinsing after use, maintaining the balance of the hydrolipidic layer of the epidermis.
Composition and properties of micellar water
The main active ingredient of the makeup remover is micelles. According to information in chemical reference books, they are fine particles in colloidal systems ranging in size from 1 to 100 nm and contain microscopic nuclei insoluble in an aqueous environment, which are surrounded by a stabilizing shell of solvent molecules and adsorbed ions.
Micelles, which make up micellar water, are part of any solution. These complex fatty acids at a certain concentration in water are surfactants, the main effect of which is:
- connection with the smallest particles of fat;
- dissolving cosmetics (lipstick, mascara, foundation, powder, blush, pencil, etc.);
- reducing the irritating effects of cleanser ingredients on the skin;
- “binding” and removing dangerous chemical compounds and carcinogens.
According to experts in the field of creating cosmetics, micellar water is needed for gentle cleansing of the skin. Active micelles, like a natural magnet, dissolve and bind particles of cosmetics and fat accumulation on the skin, after which they are removed with the ease of a magic wand with a regular cotton swab or disk.
With proper use of the product, the lipid structures of the epidermis remain intact, and if the micellar contains additional natural components (plant extracts), the upper layer of the skin is saturated with nutritional compounds.
What is the difference between micellar water and toner?
Using micellar water not only carries out a daily makeup removal procedure, but also to some extent tones the skin. However, this product cannot fully replace a tonic, since it normalizes the pH balance, and not only helps stimulate the tone of the dermis.
That is, the main difference between micellar and toner is that the first product is a cleanser, and the second is a toner, and both products are used in complex facial care. The only substitute for tonic is thermal water.
How to use micellar water correctly?

Before use, shake the bottle with liquid several times until bubbles appear on the surface. Next, a cotton pad is soaked in the composition (not too much), which is used to wipe the face along the massage lines.
To dissolve cosmetics on eyelashes and eyelids, apply the swab to closed eyes and swipe several times from top to bottom and from the nose to the temples. If desired, the face is washed or treated with a tampon soaked in ordinary or mineral water.
Do I need to wash my face after using the product?
According to the manufacturers, as indicated on the packaging, this is not necessary. In most cases, after removing makeup using micellar, you do not need to additionally wash your face with water, especially if you are in unusual or field conditions, for example, on a train or on a picnic.
However, if after using the product there is incomplete cleansing or other discomfort associated with the individual reaction of the skin, then it is better to wash your face. Some ladies prefer to carry out additional cleansing with other products with the obligatory subsequent application of a cream appropriate to the skin type and time of day.
Contraindications and precautions
As practice shows, dry and normal skin responds best to the use of micellar. On an oily face, especially prone to breakouts, an invisible film may form that does not contribute to the feeling of freshness and cleanliness. Therefore, the product is used as the first stage of complete cleansing, after which foam or gel should be used.
Always carefully read the ingredients of a cosmetic product. Budget versions of micellar water may contain various components that are not inherent in this product. Pay close attention to added extracts, especially if you have allergic reactions to herbal products. Give preference to proven product brands that you trust or consult with professionals.
Do you already use this product in your daily skin care? Share your feelings about using micellar water and tell us which cosmetic company’s product is most suitable for you, so that our readers have complete information on this issue. I wish you radiant beauty at any age!



