Free oxidation occurs with the participation of free radical forms of oxygen, which are formed in the process of one-electron reduction of oxygen and, above all, superoxide anion oxygen.
Typically, these radical oxidation reactions occur in the active center of the corresponding enzymes, and intermediate products do not appear in the external environment. When the operating conditions of the respiratory chain change (for example, during hypoxia), one-electron reduction of oxygen is also possible in it, due to the fact that its affinity for ubiquinone is higher than for cytochrome oxidase. These processes lead to the formation of superoxide anion of oxygen. This radical can also be formed under the influence of ultraviolet rays, as well as through the interaction of oxygen with metal ions of variable valency (most often with iron) or during the spontaneous oxidation of certain compounds, such as dopamine. Finally, it can be produced in cells by enzymes such as xanthine oxidase or NADPH oxidase.
The formation of superoxide anion of oxygen has important biological significance. It is a highly reactive compound, which, due to its high hydrophilicity, cannot leave the cell and accumulates in the cytoplasm. Its transformations lead to the formation of a number of active oxidizing agents (Fig. 9.10). It is capable of activating NO synthase, which forms a NO radical in tissues, which has the properties of a second messenger (activates soluble guanylate cyclase, the product of which, cGMP, exhibits vasodilator properties). On the other hand, superoxide anion is able to reduce the content of NO radical, converting it into peroxynitrite ONOOH (see Fig. 9.10).
Living cells have defense systems against increased production of free radicals. Enzyme superoxide dismutase converts the superoxide anion of oxygen into the less reactive and more hydrophobic hydrogen peroxide H2ABOUT2. Hydrogen peroxide is a substrate for catalase and glutathione-dependent peroxidases, which catalyze its conversion into a water molecule. However, hydrogen peroxide can generate a hydroxyl radical in the presence of ferrous iron or be converted into the hypochlorite anion OCl by the enzyme myeloperoxidase.
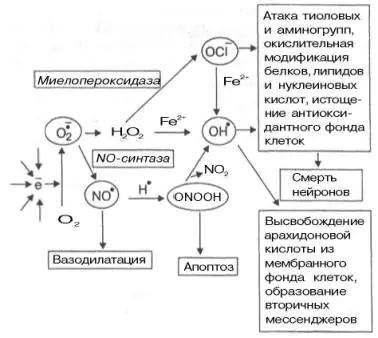
Rice. 9.10. Interconversions of free radicals and their main functions in tissues [Boldyrev A.A., 1996].
Both the hypochlorite anion and the hydroxyl radical are strong oxidizing agents. They are able to modify proteins, nucleic acids, induce lipid peroxidation (from which polyunsaturated membrane lipids “suffer” the most) and, as a result of chain reactions, lead to multiple membrane damage and cell death. An important addition to these reactions is the ability of the NO radical, when interacting with superoxide anion, to form peroxynitrite, which can induce so-called apoptosis (programmed cell death), and during its spontaneous decomposition, turn into a hydroxyl radical. The latter can also be formed from hypochlorite anion in the presence of iron ions.
The processes occurring before the formation of hypochlorite anion or hydroxyl radical are localized in the cytoplasm and controlled by cytoplasmic enzymes or natural water-soluble antioxidants. For example, taurine capable of binding hypochlorite anion in the form of a chloramine complex, a dipeptide carnosine and its derivatives neutralize the hydroxyl radical, and compounds such as protein ferritin, bind iron. Lipid peroxidation, initiated in the hydrophobic space of cell membranes, is able to interrupt the well-known hydrophobic antioxidant α-tocopherol (vitamin E). Its high concentration in biological membranes prevents them from being damaged by free radicals.
Complete suppression of peroxide processes in tissues is apparently impractical; free radicals have beneficial properties. They induce apoptosis and participate in the formation of cellular immunity. The formation of hydroperoxides of the fatty acid chains of polyunsaturated phospholipids damages the bilayer and, by stimulating the work of phospholipases, promotes the release of fatty acids from membrane lipids. Polyunsaturated arachidonic acid is a common target for free radical attack. This process can stimulate its enzymatic transformations in one of two ways - lipoxygenase or cyclooxygenase. As a result, important biological regulators are formed in the cell: prostaglandins, leukotrienes, thromboxanes. Lysophospholipids formed during the cleavage of a modified fatty acid can be restored to their original state using another fatty acid (in the form of acyl-CoA). In this way, the fatty acid composition of lipid molecules in the cell membrane can be regulated.
Highly reactive oxygen free radicals, characterized by high oxidation potential and the ability to undergo rapid transformations, can induce chain reactions. Currently, the important role of free radical processes in the development of age-related and pathological conditions in tissues is recognized [Vladimirov Yu.A. et al., 1983]. Free radical transformations are involved in mechanisms that increase cell survival under unfavorable conditions, and a decrease in the generation of free radicals in the body contributes to the weakening of cellular immunity. However, increased generation of free radicals accompanies pathological conditions (Parkinson's disease, Alzheimer's disease) and the process of biological aging itself.
LEVEL OF FREE RADICALS GENERATION IN EJACULATE SAMPLES OF INFERTILITY PATIENTS
The level of generation of free radicals in ejaculate samples of infertile patients was assessed using the chemiluminescence method. It has been shown that in samples containing antisperm antibodies, the likelihood of damage to the plasma membrane of sperm is increased due to excessive generation of free radicals. Much attention is paid to the study of the role of antisperm antibodies (ASAT) in the reproductive process. However, the question of the effect of ACAT on fertilization still remains unclear. The works of some authors reveal a relationship between the presence of antibodies and a decrease in the likelihood of pregnancy, while in other studies the influence of ACAT on the decrease in this indicator in patients with antibodies is questioned. The purpose of this work was to assess the level of SR generation in ACAT-positive and ACAT-negative ejaculate samples.
Publication: Bulletin of Experimental Biology and Medicine
Year of publication: 2001
Volume: 3s.
Additional information: 2001.-N 6.-P.658-660
Views: 171
Reactive oxygen species (ROS) – compounds in which oxygen has an unpaired electron.
ROS are formed when the operating conditions of the respiratory chain change (for example, during hypoxia), under the influence of UV rays, during the interaction of oxygen with metal ions of variable valence (iron), during the spontaneous oxidation of certain substances, with the participation of the enzymes xanthine oxidase or NADPH oxidase. Under these conditions, it is formed superoxide anion oxygen O2 .− , then hydrogen peroxide H2ABOUT2 And hydroxide radical HO. . They cause reactive oxygen species lipid peroxidation - a process leading to severe membrane damage, damaging proteins and DNA.
Inactivation of reactive oxygen species in cells occurs under the action of the antioxidant system. It includes several antioxidant enzymes and low molecular weight antioxidants (vitamin C, glutathione, vitamin E, etc.).
Superoxide dismutase(SOD) converts the superoxide anion of oxygen into hydrogen peroxide H2ABOUT2:
Catalase - hemin enzyme containing Fe 3+ catalyzes the decomposition reaction of hydrogen peroxide. This produces water and oxygen:
The highest activity of catalase in the body is characteristic of the liver. There is a lot of catalase in erythrocytes. There it protects hemoglobin heme from oxidation.
Peroxidase- heme enzyme, reduces hydrogen peroxide to water; At the same time, another substance is oxidized:
Peroxidase is capable of decomposing other peroxides, converting them into alcohols. Peroxidase activity is found in the liver, kidneys, and neutrophilic leukocytes.
Antioxidants - biologically active substances that interact with free radicals and prevent the processes of free radical oxidation of organic substances in the body.
Vitamins,exhibiting antioxidant properties - S, E, A, R. Tripeptide exhibits antioxidant properties glutathione, taurine (2-aminoethanesulfonic acid), dipeptide carnosine
Complete suppression of peroxide processes in tissues is apparently impractical. Free radicals induce apoptosis, participate in the formation cellular immunity, stimulate the work of phospholipases, thereby participating in the synthesis of eicosanoids.
However, increased generation of free radicals accompanies pathological conditions (Parkinson's disease, Alzheimer's disease) and the process of biological aging itself.
Date added: 2015-03-19; views: 759; ORDER A WORK WRITING



